Abstract
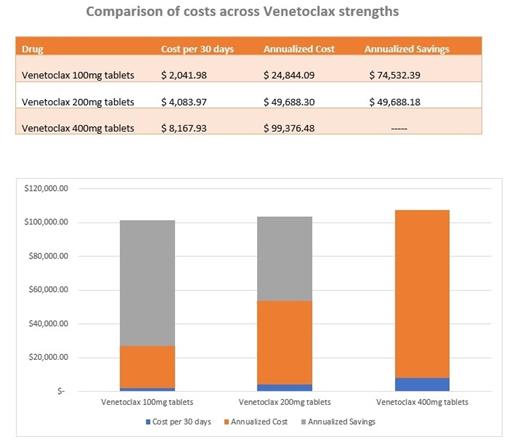
Venetoclax is a potent selective inhibitor of the anti-apoptotic protein B-Cell Lymphoma 2 (BCL2) on tumor cells restoring the process of apoptosis among cancer cells. Venetoclax has been approved by the US Food and Drug administration (FDA) for the treatment of patients with chronic lymphocytic leukemia (CLL) with 17p deletion (del[17p]) who received at least one prior therapy. CLL is the most common leukemia in older adults and its treatment is often challenging given the multiple comorbidities, impaired mobility, and poor functional reserved affecting this patients' population. Emerging targeted therapy for relapsing CLL such as Venetoclax has provided hope, however, there is limited data regarding its efficacy and safety in the geriatric population. Although, a recent international cohort study showed that Venetoclax had similar efficacy in the elderly, those older than 75 years had a higher rate of treatment discontinuation due to toxicity which can lead to significant complications, multiple hospitalizations, and increased healthcare costs. For veterans, the annual treatment cost using the manufacturer-recommended dose of Venetoclax is close to $100,000. As it is common to reach complete remission with Venetoclax monotherapy, patients most likely will remain on therapy long-term, compounding financial impact over their treatment lifetime. Reduced dose Venetoclax can lower total cost by 50% or more thereby decreasing the economic burden, which would ultimately improve treatment adherence. In addition, Venetoclax dose reductions could potentially allow disease control while decreasing the rate and intensity of side effects and adverse events in frail older adults. Therefore, we present our experience using a reduced Venetoclax dose, which led to complete remission in the treatment of two frail older adults with relapsing CLL at the Washington DC Veterans Affairs Medical Center. We report the disease control as evidenced by decrease in white blood cell count and alleviation of "B symptoms" along with side effects with a Venetoclax dose ranging from 100-200 mg per day. We also report the cost benefits obtained with the use of a reduced Venetoclax dose. We hope that sharing our experience encourages clinical trials aiming to determine the minimal dose of Venetoclax needed to effectively treat CLL while limiting toxicity, decreasing health care costs, and preserving quality of life in frail older adults.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal